Recombinant Proteins
Recombinant protein technology is formed by cloning a foreign recombinant gene into a vector (usually a plasmid) and then expressing the corresponding protein in the host cell. In order to achieve this goal, at first the desired structure is designed and the target gene is cloned into the appropriate plasmid and then it is introduced into the host cell. In some cases, additional sequences (such as initiation or translation signals) may be required to induce expression of the recombinant DNA. In the next step, expression takes place and the recombinant protein is made.
In general, prokaryotic and eukaryotic systems can be used to produce recombinant proteins. Choosing the right system for recombinant protein production is based on the type, structure and activity of the desired protein. In the bacterial expression system, which is one of the most widely used expression systems, it is possible to produce large amounts of recombinant protein at a high scale and at a low cost. Although the amount of recombinant protein expression in the eukaryotic system is lower than the bacterial system, but since the post-translational changes such as glycosylation in these systems are very close to human cells, in the materials that are aimed at producing human medicine, eukaryotic systems and especially mammalian cells are the preferred choice.
The general process of producing recombinant proteins in bacteria includes, primarily, a plasmid structure is designed and made, and after approval, transformed into bacteria (usually E.coli sushi). Then, protein expression is induced by creating the desired conditions when the bacterial density reaches the appropriate level (for example, by adding isopropyl beta-di-1-thiogalactopyranoside or IPTG in laboratory conditions) and after performing the fermentation process, it is harvested and purified by the protein method properly. In order to produce recombinant proteins in the eukaryotic system, the confirmed plasmid is transfected into the host cell with the help of a suitable substance (Transfection Reagent) and after the required time has passed (which is usually longer than that of bacteria), sufficient expression of the protein takes place, which is harvested and purified. These steps are shown schematically in Figure 1.
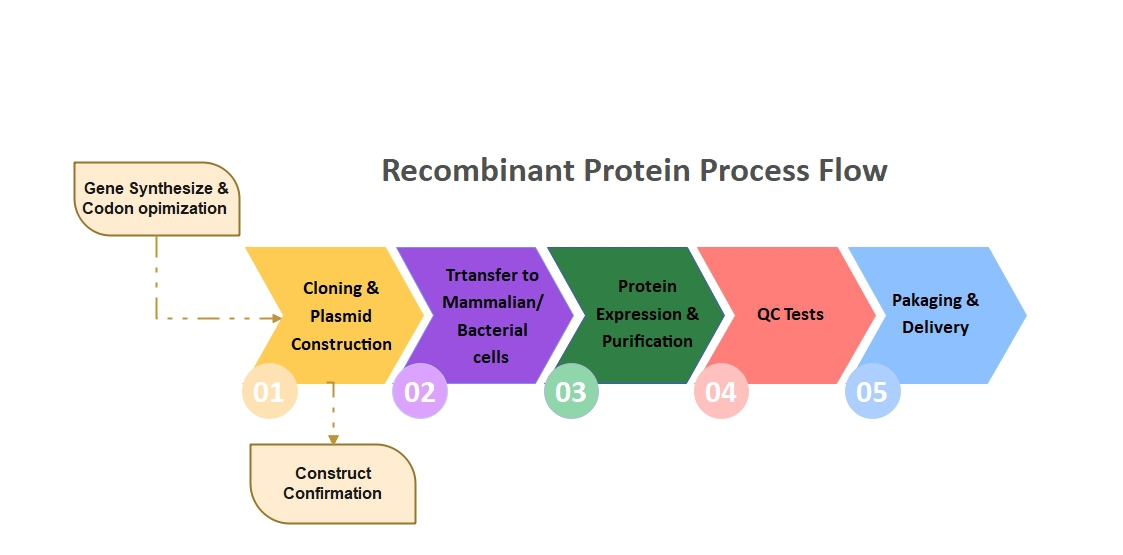
Figure 1. Recombinant protein production process
With years of experience in genetic engineering and biotechnology, Hum-Immune Biotech research team has the ability to design, produce, purify and quality control of recombinant proteins in both prokaryotic and eukaryotic expression systems. Our company is ready to provide services in this field on a laboratory and semi-industrial scale.
